
Drug Target Models
GPCR Reporter Cells
Immunotherapy cells
Other Stable Cells
Assay Kits & Reagents
Services
Resources
Company
Committed to providing comprehensive support for global drug development
CHO-K1 Human CCR7 β-Arrestin Cell Line
Cat. No: RQP71399
Size: 1 vial of frozen cells (>1E6 per vial in 1 mL)
Unit Price: Contact For Pricing
Product Info
Biological Information
Assay Data
Cell Culture
| Cat. No | RQP71399 |
| Product Name | CHO-K1 Human CCR7 β-Arrestin Cell Line |
| Product Type | Receptor Cell Lines |
| Product Description | CHO-K1 Human CCR7 β-Arrestin Cell Line is a clonally stable cell line constructed using lentiviral technology,constitutively expressing the Human CCR7 gene. |
| Culture Properties | Adherent |
| Stability | 32passages (in-house test, that not means the cell line will be instable beyond the passages we tested.) |
| Mycoplasma Status | Negative |
| Culture Medium | N/A |
| Freeze Medium | 90% FBS+10% DMSO |
| Storage Conditions | Liquid nitrogen immediately upon delivery |
| Transducer | β-Arrestin |
| Application | Functional assay for CCR7 |
For research use only. Not intended for human or animal clinical trials, therapeutic or diagnostic use.
| Target Class | GPCR |
| Family | C-C motif chemokine receptors |
| Sub Family | Class A(Rhodopsin) |
| Gene Name | CCR7 |
| Gene Aliases | CMKBR7;EBI1;BLR2;CDw197;CD197 |
| Gene ID | 1236 |
| Accession Number | NM_001838.4 |
| UniProt Number | P32248 |
| Protein Name | C-C CKR-7; CC-CKR-7; CCR-7 |
| Protein Aliases | BLR2;CDw197; Epstein-Barr virus-induced G-protein coupled receptor 1 (EBI1; EBV-induced G-protein coupled receptor 1);MIP-3 beta receptor |
| Target Species | Human |
| Host cell | CHO-K1 |
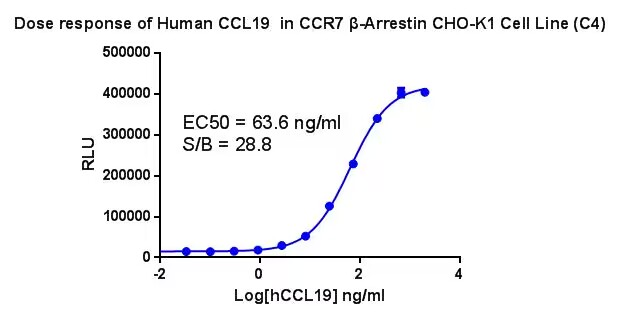
Figure 1. Dose response of Human CCL19 in CCR7 β-Arrestin CHO-K1 Cell Line (C4).
Cell Resuscitation
1)Rapidly thaw the frozen cells in a 37 °C water bath for approximately 60 seconds. Once thawed (which may take slightly less or more than 60 seconds), immediately transfer the cell suspension from the cryovial into a 15 mL centrifuge tube containing 10 mL of pre-warmed CHO-K1 Human CCR7 β-Arrestin Cell Line complete culture medium.
2)Centrifuge cells at 1000 rpm for 5 min to remove medium, then resuspend cells in 5 mL of pre-warmed complete medium.
3)Transfer the cell suspension into a T25 culture flask and incubate at 37 °C with 5% CO₂.
4)After approximately 24–36 hours, replace the medium or passage the cells to remove non-adherent dead cells.
Subculturing procedure
1)When the cell density reaches the appropriate confluency for passaging, wash the cells with PBS, then add 1 mL trypsin to detach the cells. When more than 80% of the cells detach upon gently tapping the culture flask, add complete culture medium to terminate digestion. Gently pipette to obtain a single-cell suspension, transfer to a 15 mL centrifuge tube, and centrifuge at 1000 rpm for 5 minutes.
2)Discard supernatant after centrifugation. Resuspend cells in fresh medium to a single-cell suspension and transfer to a new culture flask for continued growth.
Cell Freezing
After trypsinization and centrifugation of cells from each T75 flask or 10 cm culture dish, discard the supernatant. Add 2 mL of cryopreservation medium (90% FBS + 10% DMSO), gently resuspend thoroughly, and aliquot into two cryovials. Immediately place the cryovials into a controlled-rate freezing container (e.g., Nalgene 5100-0001), fill with isopropanol to the indicated level, and store at −80 °C. After 24 hours, transfer the cryovials to liquid nitrogen for long-term storage.
Related products
CHO-K1 Human Gα15(GNA15) Cell Line
CHO-K1 Human HCAR1 Cell Line
HEK293 Human CALCRL&RAMP3 CRE-Luc
CHO-K1 Human 5-HTR2C(no edited) Receptor Cell
CHO-K1 Human 5-HTR2C (edited) Cell Line
CHO-K1 Human RXFP1 Cell Line
HEK293 Human CaSR NFAT-Luc Cell Line
HEK293 Human LHCGR CRE-Luc Cell Line
HEK293 Human PTGER4 Cell Line
HEK293 Human 5-HTR2A Cell Line
We Are Pleased to Announce: Global Commercial Licensing Rights for Jurkat E6.1, CHO-K1, and HEK293 Cell Lines Officially Secured.
Explore