
Drug Target Models
GPCR Reporter Cells
Immunotherapy cells
Other Stable Cells
Assay Kits & Reagents
Services
Resources
Company
Professional technology integration to support the entire R&D process
Lentiviral Integration
Product Description
Cycle Pricing
Case Studies
Definition
Lentivirus:
Lentivirus is a genus of retroviruses within the Retroviridae family. Lentiviruses encode reverse transcriptase and integrase, enabling viral RNA to be reverse-transcribed into DNA and stably integrated into the host genome. Following integration, the viral genetic material is replicated during host cell division, allowing for long-term and stable gene expression..
Unlike other retroviruses that primarily infect dividing cells, lentiviruses can efficiently transduce both dividing and non-dividing cells, making them widely used in gene delivery and cell engineering applications.
Lentiviral vectors:
Wild-type lentiviruses have been engineered into replication-deficient lentiviral vectors for safe laboratory use. These vectors retain key advantages, including broad cell tropism, stable transgene expression, and low immunogenicity.
The native lentiviral genome is approximately 8–10 kb in length and consists of single-stranded RNA. For experimental applications, essential viral components required for replication have been removed or modified to enhance biosafety.
The native lentiviral genome is approximately 8–10 kb in length and consists of single-stranded RNA. For experimental applications, essential viral components required for replication have been removed or modified to enhance biosafety.
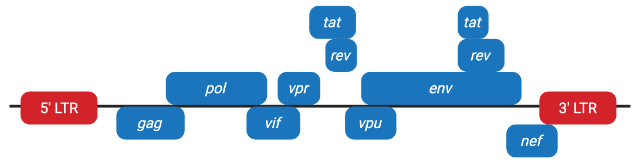 Figure 1. Schematic representation of the complete lentiviral genome.
Figure 1. Schematic representation of the complete lentiviral genome.1.Packaging genes include: gag, pol, env:gag、pol、env
2.Regulatory genes include: tat, rev:tat、rev
3.Accessory genes include: vif, vpr, vpu, nef
2.Regulatory genes include: tat, rev:tat、rev
3.Accessory genes include: vif, vpr, vpu, nef
| Transfer plasmid | LTR | in cis | Long terminal repeat; comprised of a U3-R-U5 structure and are found on each side of the provirus. The U3 (unique 3’) contains sequences necessary for activation of viral genomic RNA transcription. R is the repeat region. |
|
U3 |
in cis | Unique 3’; contains sequences necessary for activation of viral genomic RNA transcription. Removal of this region in the 3’ LTR in third-generation plasmids creates self-inactivating viral vectors. | |
| R | in cis | Repeat region where Tat binds. | |
|
TAR
|
in cis | Trans-activating response element; found in the R region and acts as the binding site for Tat. | |
| U5 | in cis | Unique 5'; in third-generation plasmids, this region is often removed in 5’ LTRs and replaced with a heterologous promoter (CMV or RSV). | |
| 5' LTR | in cis | Acts as an RNA pol II promoter; the transcript begins, by definition, at the beginning of R, is capped, and proceeds through U5 and the rest of the provirus. Third-generation plasmids use a hybrid 5' LTR with a constitutive promoter such as CMV or RSV. | |
| 3' LTR | in cis | Terminates transcription started by 5' LTR by the addition of a polyA tract just after the R sequence. | |
| WPRE | in cis | Woodchuck hepatitis virus post‐transcriptional regulatory element; stimulates the expression of transgenes via increased nuclear export. | |
| RRE | in cis | Rev Response Element; he sequence to which the Rev protein binds. | |
| Psi (Ѱ) | in cis | RNA packaging signal; recognized by nucleocapsid proteins and essential for efficient viral packaging. | |
| cPPT | in cis | Central polypurine tract; recognition site for proviral DNA synthesis. Increases transduction efficiency and transgene expression. | |
| Packaging plasmid | gag | in trans | Precursor structural protein of the lentiviral particle containing matrix, capsid, and nucleocapsid components. |
| pol | in trans | Precursor protein containing reverse transcriptase and integrase components. | |
| rev | in trans | Binds to the Rev Response Element (RRE) within unspliced and partially spliced transcripts to facilitate nuclear export. Provided by a separate plasmid from gag/pol in third-generation packaging plasmids. | |
| tat | in trans | Trans-activator; binds TAR to activate transcription from the LTR promoter. Only in first- and second-generation lentiviral plasmids. | |
| Envelope plasmid | env | in trans | The viral envelope gene; typically vesicular stomatitis virus G glycoprotein (VSV-G), an envelope protein with broad tropism used to pseudotype most lentiviral vectors. |
The Evolution of Lentiviral Vectors
To improve biosafety in laboratory lentivirus production, non-essential viral components are removed, and essential elements are distributed across multiple plasmids.
This design strategy has resulted in four widely used generations of lentiviral packaging systems:
This design strategy has resulted in four widely used generations of lentiviral packaging systems:
First-generation plasmids include (Figure 2):
1. Transfer plasmid—contains the transgene and wild-type LTR
2. Packaging plasmid—contains the entire viral genome (packaging, regulatory, and accessory genes), minus only the envelope
3. Envelope plasmid—contains the env.
1. Transfer plasmid—contains the transgene and wild-type LTR
2. Packaging plasmid—contains the entire viral genome (packaging, regulatory, and accessory genes), minus only the envelope
3. Envelope plasmid—contains the env.
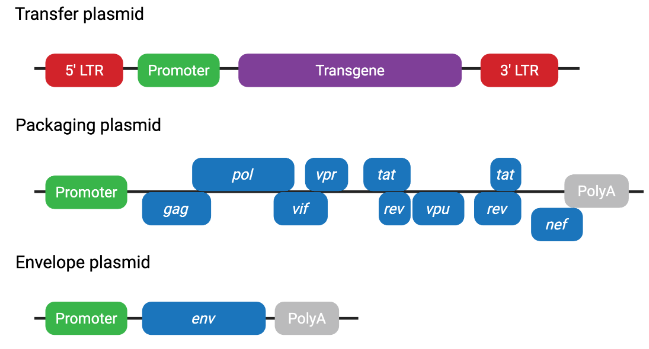 Figure 2. First-generation lentiviral plasmid system
Figure 2. First-generation lentiviral plasmid systemFirst-generation lentiviral plasmids have largely been phased out due to limited biosafety improvements and the potential risk of generating replication-competent lentivirus (RCL).
Second-generation plasmids include (Figure 3):
1. Transfer plasmids—containing the transgene and wild-type LTR
2. Packaging plasmids—containing gag, pol, tat, and rev
3. Envelope plasmids—containing env
Second-generation plasmids include (Figure 3):
1. Transfer plasmids—containing the transgene and wild-type LTR
2. Packaging plasmids—containing gag, pol, tat, and rev
3. Envelope plasmids—containing env
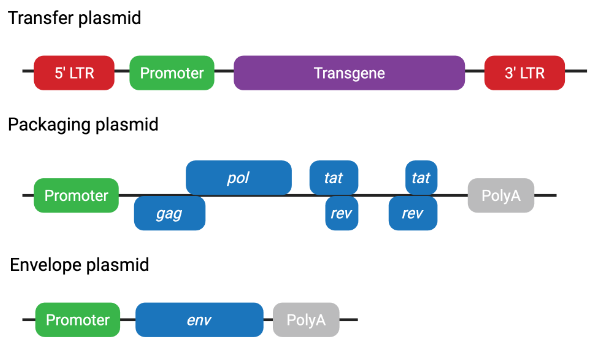
Figure 3 Second-generation lentiviral plasmid system
Second-generation lentiviral plasmids improve biosafety by removing non-essential accessory genes. Tat is still required in this system, as transcription from the 5’LTR of the transfer plasmid relies on Tat-mediated activation.
Third-generation plasmids include (Figure 4):
1. Transfer plasmid—contains the transgene and LTR (chimeric 5' LTR)
2. Packaging plasmid 1—contains gag and pol
3. Packaging plasmid 2—contains rev
4. Envelope plasmid—contains env
1. Transfer plasmid—contains the transgene and LTR (chimeric 5' LTR)
2. Packaging plasmid 1—contains gag and pol
3. Packaging plasmid 2—contains rev
4. Envelope plasmid—contains env
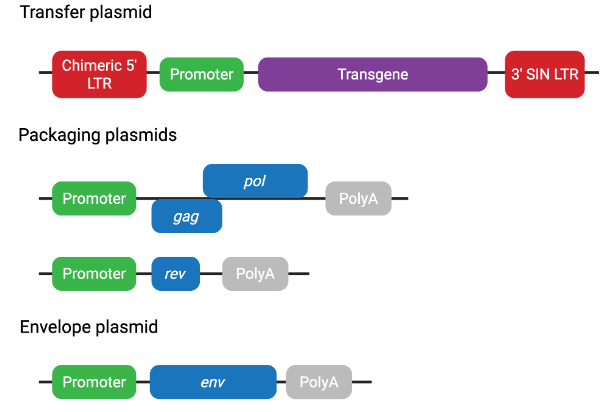
Figure 4 Third-generation lentiviral plasmid systems
Third-generation lentiviral systems further enhance biosafety through additional genetic separation and regulatory modifications. The packaging components are split across two plasmids, reducing the risk of recombination but increasing system complexity, which may result in lower viral titers.
Unlike second-generation systems, third-generation systems are Tat-independent. The transfer plasmid contains a chimeric 5’ LTR driven by a heterologous promoter, typically CMV or RSV, eliminating the need for Tat-mediated transactivation.
Most third-generation transfer plasmids also incorporate a deletion in the 3’ LTR, creating a self-inactivating (SIN) configuration. This deletion is copied to the 5’ LTR during reverse transcription, thereby preventing transcription of full-length viral RNA after genomic integration. This phenomenon is also common with second-generation transfer plasmids.
Most third-generation transfer plasmids also incorporate a deletion in the 3’ LTR, creating a self-inactivating (SIN) configuration. This deletion is copied to the 5’ LTR during reverse transcription, thereby preventing transcription of full-length viral RNA after genomic integration. This phenomenon is also common with second-generation transfer plasmids.
Fourth-generation plasmids include (Figure 5):
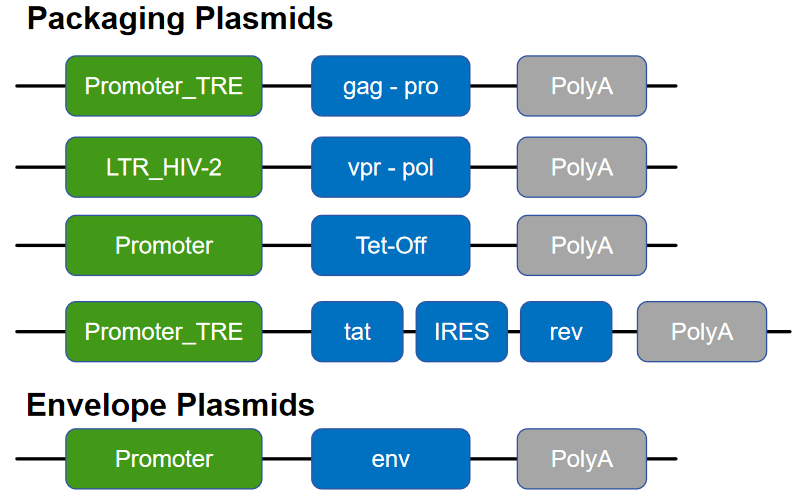
Figure 5 Fourth-generation lentiviral plasmid system
Fourth-generation lentiviral packaging systems are optimized for viral yield, ease of use, and enhanced biosafety. These systems incorporate tetracycline-inducible promoters to enable controlled transcriptional activation, while retaining Tat to achieve high-level expression of essential viral components. To further reduce the risk of recombination, key viral elements such as gag-pro and vpr-pol are separated across multiple plasmids, increasing genetic separation and minimizing the likelihood of replication-competent lentivirus (RCL) formation. This design enables efficient lentivirus production while maintaining a high level of biosafety. Unconcentrated viral supernatants produced using fourth-generation systems have been reported to reach titers of up to 5 × 10⁸ IFU/mL.
In a summary, through successive design improvements, fourth-generation lentiviral packaging systems have become widely adopted in laboratory settings, offering an optimal balance of safety, usability, and high viral yield.
Packaging Host Cells
1. Lentivirus packaging requires the selection of a producer cell line. HEK293T is commonly used. Compared to HEK293, HEK293T cells express the SV40 large T antigen, allowing plasmids containing the SV40 replication origin to replicate episomally, resulting in higher copy numbers. HEK293T cells also have higher transfection efficiency and support higher viral titers.
2. 293T/17 is a subclone of 293T cells that also expresses the SV40 large T antigen. Clone 17 was selected for its higher transfection efficiency in testing.
3. 293FT is also a derivative of 293T cells, a fast-growing variant that also expresses the SV40 large T antigen.
4. Lenti-X 293 cells are also a derivative of 293T cells that express the SV40 large T antigen. It has been reported that Lenti-X 293 cells can package six times more lentivirus than 293FT cells.
5. Suspension 293T cells and various suspension cell derivatives. Suspension cells can achieve higher densities and can be expanded to bioreactors. They are serum-free, facilitating downstream purification and concentration.
6. Stable production cell lines, with the necessary packaging components stably integrated into the host, simplify the production process and ensure batch stability of the virus, eliminating the need for individual packaging required for transient transfection.
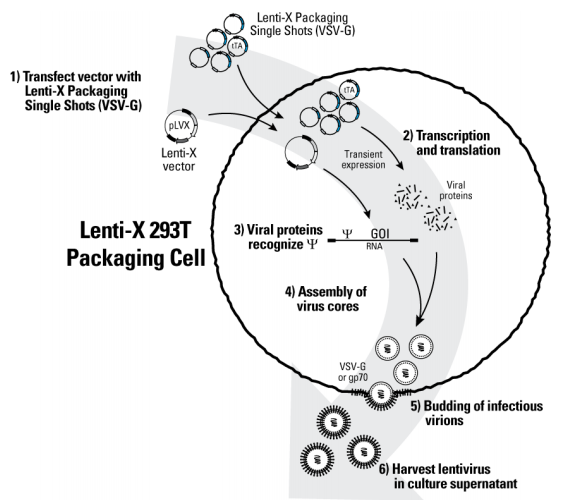
Figure 6 Lentivirus packaging diagram
Purification and Concentration
High-titer and high-purity lentiviral preparations are essential for efficient transduction of target cells. Therefore, harvested viral supernatants typically require further purification and concentration prior to downstream applications.
A variety of methods are available for lentiviral concentration and purification. The choice of approach depends on factors such as sample volume, required purity, processing time, and scalability. Commonly used methods include centrifugation-based techniques, filtration, precipitation, and affinity-based capture. Some methods, such as ultracentrifugation, provide high purity but can be time-consuming, while others, such as polyethylene glycol precipitation, are faster but may not achieve the same purity. Newer techniques, such as those using magnetic nanoparticles, allow for rapid, high-yield concentration with minimal handling.
The following is a detailed description of some key methods:
1. Centrifugation:
Differential centrifugation:
This method uses different centrifugation speeds to separate viruses from cellular debris and other components based on size and density.
Ultracentrifugation:
This technique uses extremely high speeds to pellet the virus, thereby concentrating and purifying it.
Centrifugal ultrafiltration:
This method utilizes membranes with specific molecular weight cutoffs to filter out larger particles and concentrate the virus.
2. Filtration:
Tangential flow filtration: This method uses a membrane to separate viral particles from a bulk liquid, enabling continuous flow and concentration.
Membrane adsorption/elution: Viruses can be adsorbed to specific membrane filters and then eluted using an appropriate buffer.
Tangential flow filtration: This method uses a membrane to separate viral particles from a bulk liquid, enabling continuous flow and concentration.
Membrane adsorption/elution: Viruses can be adsorbed to specific membrane filters and then eluted using an appropriate buffer.
3. Precipitation:
Polyethylene glycol (PEG) precipitation: PEG is used to precipitate viruses from solution for collection and concentration.
Coprecipitation: In some cases, viruses can be coprecipitated with other substances (e.g., proteins) to enhance concentration.
Polyethylene glycol (PEG) precipitation: PEG is used to precipitate viruses from solution for collection and concentration.
Coprecipitation: In some cases, viruses can be coprecipitated with other substances (e.g., proteins) to enhance concentration.
4. Immunocapture:
Antibody-based capture: Viruses can be captured using antibodies that specifically bind to the virus, enabling isolation and concentration.
Antibody-based capture: Viruses can be captured using antibodies that specifically bind to the virus, enabling isolation and concentration.
5. Other methods:
Magnetic nanoparticles: These nanoparticles can be used to capture and concentrate viruses, providing rapid and efficient concentration.
Acoustic separation: This method uses sound waves to separate particles based on size and density, potentially offering a gentle and effective method for viral concentration.
Water-based condensation: This newer technology is under development for sampling viruses in the air.
Titer Measurement
Viral titer refers to the concentration of infectious viral particles in a preparation and is a critical parameter for lentiviral transduction. Following concentration, viral titers are typically determined prior to infecting target cells to ensure consistent and reproducible results.Multiple methods are available for viral titer measurement, including plaque assays, focus-forming assays, and other infectivity- or particle-based quantification techniques.
1. Plaque Assay:
This method involves infecting a monolayer of host cells with serial dilutions of a viral sample.
After incubation, visible plaques (areas of cell lysis or death) form, and the number of plaques is counted.
The titer is calculated based on the number of plaques, the dilution factor, and the amount of virus used.
2. Focus Assay:
Similar to the plaque assay, this method quantifies the number of foci (areas of infected cells) formed after infection.
The titer is determined by counting foci and taking into account the dilution and volume.
3. Other Methods:
qPCR: Quantifies viral RNA or DNA, providing a measure of total viral particles.
ELISA: Detects viral antigens, providing a method for quantifying viral load.
Flow cytometry: Can be used to count single infected cells, especially those expressing fluorescent markers.
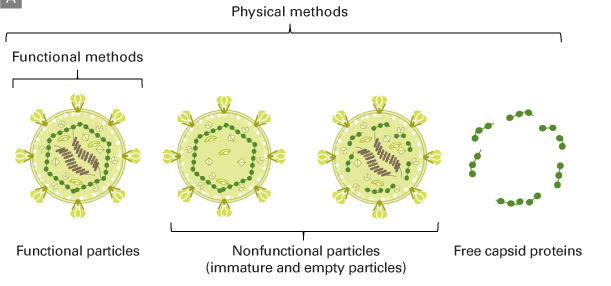
Figure 7. Products present in lentiviral packaging supernatant
Physical methods can measure functional and nonfunctional particles released into the supernatant, as well as free capsid proteins (e.g., p24). Functional titration methods specifically measure functional viral particles.
Important points to note:
1. Infectious titer vs. physical titer:
Some methods measure the number of infectious viral particles (infectious titer), while others measure the total number of viral particles (physical titer). Infectious titer better reflects the virus's ability to infect and transduce. However, detecting infectious titer generally takes longer, while detecting physical titer is faster. Therefore, one type of method establishes a conversion relationship between "infectious titer" and "physical titer."
1. Infectious titer vs. physical titer:
Some methods measure the number of infectious viral particles (infectious titer), while others measure the total number of viral particles (physical titer). Infectious titer better reflects the virus's ability to infect and transduce. However, detecting infectious titer generally takes longer, while detecting physical titer is faster. Therefore, one type of method establishes a conversion relationship between "infectious titer" and "physical titer."
2. What is the difference between plaque-forming units (PFU), transduction units (TU), and infectious units (IFU)? Which one better reflects the amount of active virus used?
1) PFU (plaque-forming unit) indicates the number of infectious or live virus. PFU/ml is used to calibrate viral titer by measuring the number of plaques formed after virus infection and reflects the amount of active virus in the preparation.
2) IFU (infectious unit) is equivalent to PFU.
3) TU/ml: transduction units/ml, indicates the number of biologically active viral particles per milliliter of viral venom, i.e., the number of viral genomes capable of infecting and entering target cells. This is a commonly used unit of measurement for lentiviruses and reflects the amount of working virus in a preparation.
The number of IFU is typically lower than the number of TU because not all viral particles that enter cells successfully initiate productive infection.
1) PFU (plaque-forming unit) indicates the number of infectious or live virus. PFU/ml is used to calibrate viral titer by measuring the number of plaques formed after virus infection and reflects the amount of active virus in the preparation.
2) IFU (infectious unit) is equivalent to PFU.
3) TU/ml: transduction units/ml, indicates the number of biologically active viral particles per milliliter of viral venom, i.e., the number of viral genomes capable of infecting and entering target cells. This is a commonly used unit of measurement for lentiviruses and reflects the amount of working virus in a preparation.
The number of IFU is typically lower than the number of TU because not all viral particles that enter cells successfully initiate productive infection.
Infecting Target Cells
Lentiviral transduction involves integrating the target gene into the host genome. This is achieved by modifying the reverse transcriptase and integrase enzymes encoded within the lentiviral vector, enabling sustained expression of the exogenous gene. Unlike other viral vectors, lentiviruses can transduce non-dividing cells, which offers unique advantages in gene delivery applications.
In establishing stable cell lines, lentiviruses infect host cells. The reverse-transcribed DNA remains partially free in the cells, while the integrase enzyme integrates the 5' to 3' LTR sequences of the lentiviral vector into the host genome. Under selective resistance screening, non-integrated cells are killed, and surviving cells can be expanded to establish stable cell lines. Studies have shown that it takes approximately 10-14 days for the non-integrated DNA to be completely degraded as cells divide, which is also the period of pressure screening for resistance.
Lentiviruses (LVs) are enveloped viruses, the most common of which is VSV-G. They enter cells by binding to specific cell surface proteins (such as LDLR), then undergoing membrane fusion and injecting viral RNA containing their genetic information into the cytoplasm. This single-stranded viral RNA is converted into DNA by reverse transcription. The viral DNA enters the cell nucleus and is integrated into the host cell genome by the viral integrase.
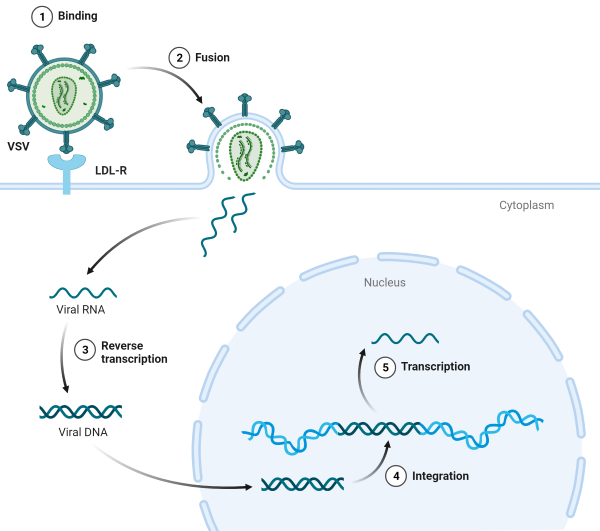
Figure 8 Schematic diagram of lentivirus integration into the host genome
Key Considerations in Experiments
1. MOI Selection: An MOI that is too low will result in low transduction efficiency, while an MOI that is too high may cause cytotoxicity. MOI titration experiments are necessary to determine optimal infection conditions.
2. Polybrene Use: Polybrene is a polycation that reduces charge repulsion between the virus and the cell membrane, thereby improving transduction efficiency. However, if the dosage is inappropriate, it may also cause cell damage.
3. Timing: Transduction should be performed as soon as cell viability has recovered, ensuring that cells are in an active proliferation phase as soon as possible.
4. Cell Density Control: Excessively high cell density increases transduction costs, while excessively low density may affect efficiency. It is recommended to maintain a cell density of approximately 1-2 × (10^5) cells/mL.
5. Viral Concentration and Titer: Lentivirus titer directly affects transduction efficiency, emphasizing the importance of understanding viral titer and controlling viral addition.
6. Integration: Genome-wide studies of viral integration have shown that lentiviruses most frequently integrate into transcriptionally active regions, regions recently involved in translocation events, and other "vulnerable" genomic locations, and this preference is conserved across target species. While the general tendency toward integration is known, it remains random and difficult to predict.
Finally:
Due to their ability to stably integrate into the host genome and support long-term transgene expression, lentiviral vectors are widely used in both research and clinical settings. Common applications include stable cell line generation, CRISPR library delivery, animal model development, and gene therapy research. Lentiviral transduction provides a reliable and versatile gene delivery approach across a broad range of cell types, including non-dividing cells that are often refractory to other transduction methods. Careful experimental design and optimization can significantly enhance transduction efficiency and the stability of gene expression.
Stable Cell Line Construction Services
Reqbio offers stable cell line construction services based on a “lentiviral Integration System”. With a proven track record of over 900 successfully generated stable cell lines, we bring substantial expertise to support diverse research and development needs. Please contact us to discuss your project requirements.
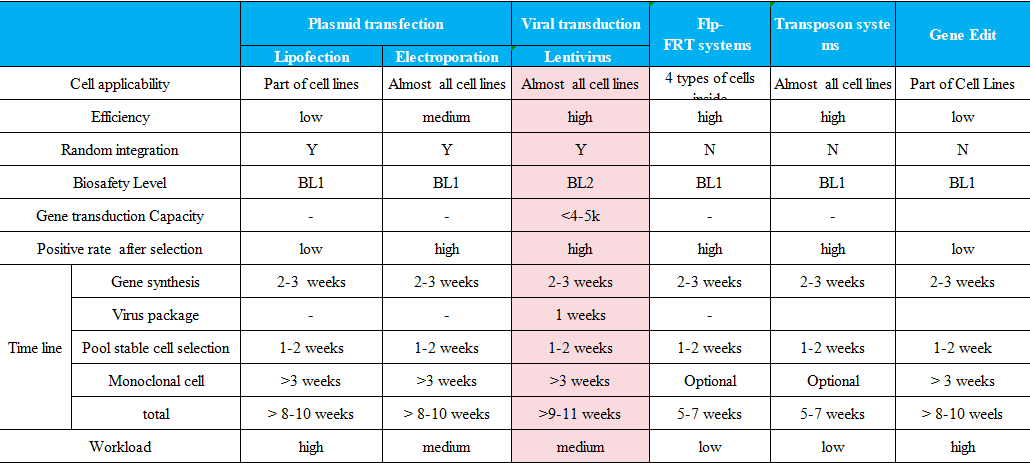
Other stable cell lines
Plasmid Electroporation
Flp-FRT Targeted Integration
Random Plasmid Integration
Transposon-Based Integration
If you are interested in ordering, please contact us.
Customer help-line
4008-750-250
sales@reqbio.com
Office address:
3rd Floor, No. 6, Lane 222, Guangdan Road, Pudong New Area, Shanghai, China
We Are Pleased to Announce: Global Commercial Licensing Rights for Jurkat E6.1, CHO-K1, and HEK293 Cell Lines Officially Secured.
Explore